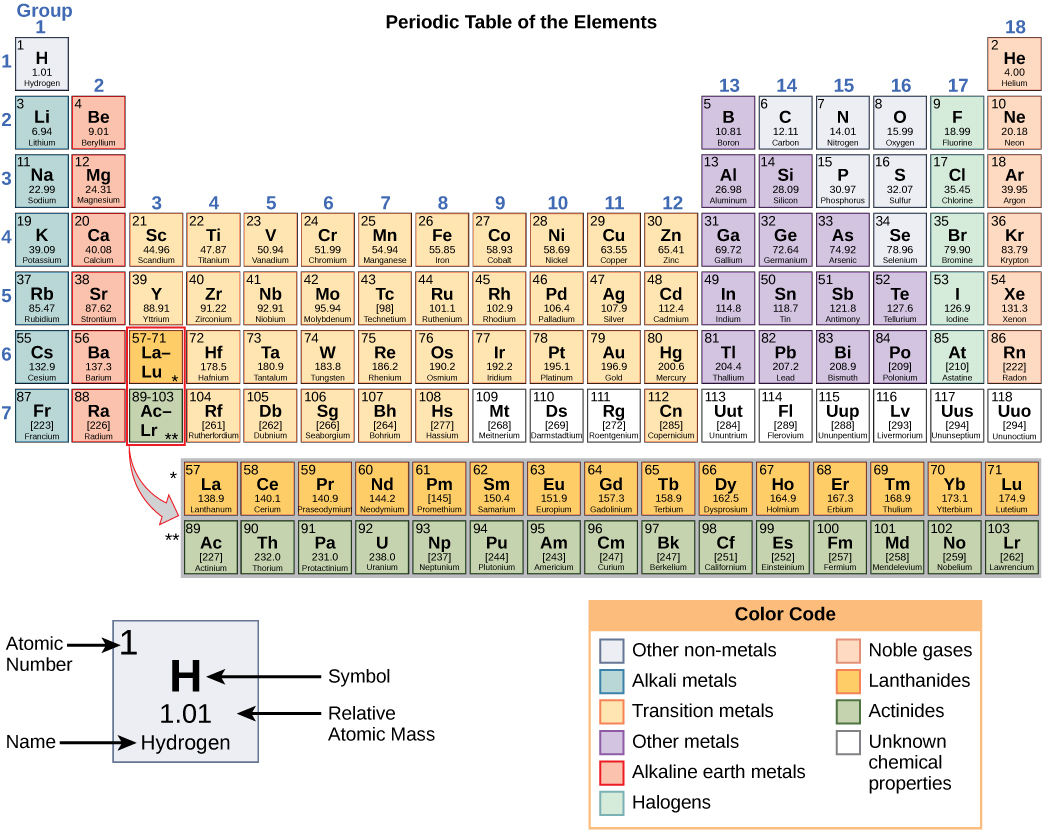
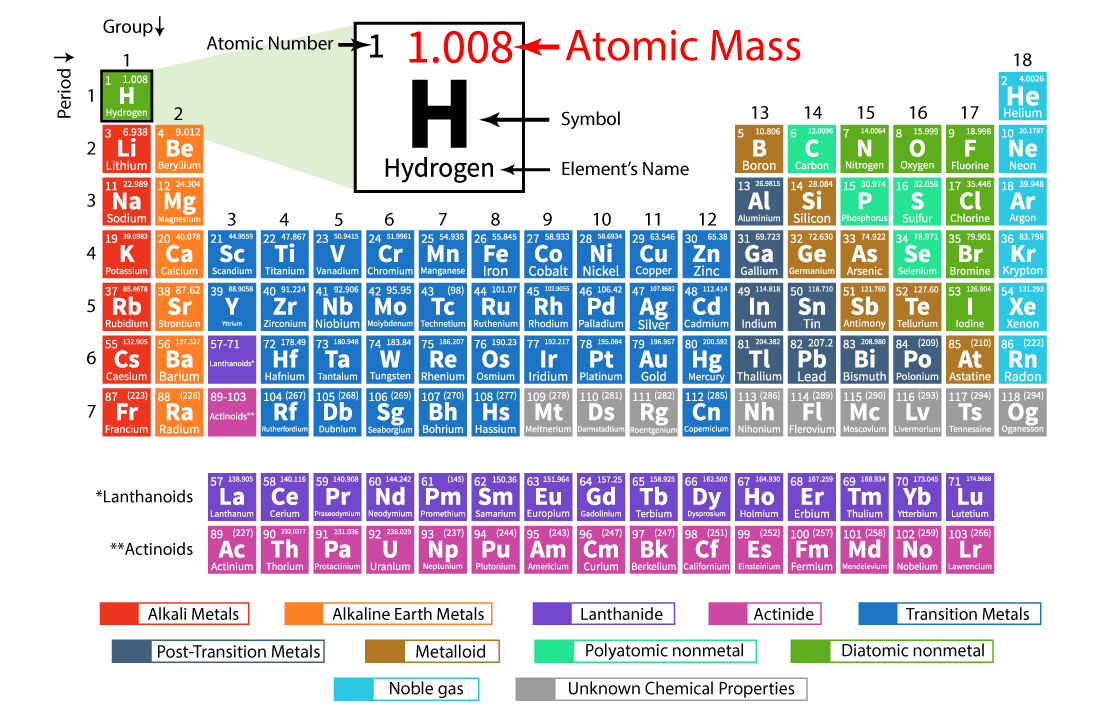
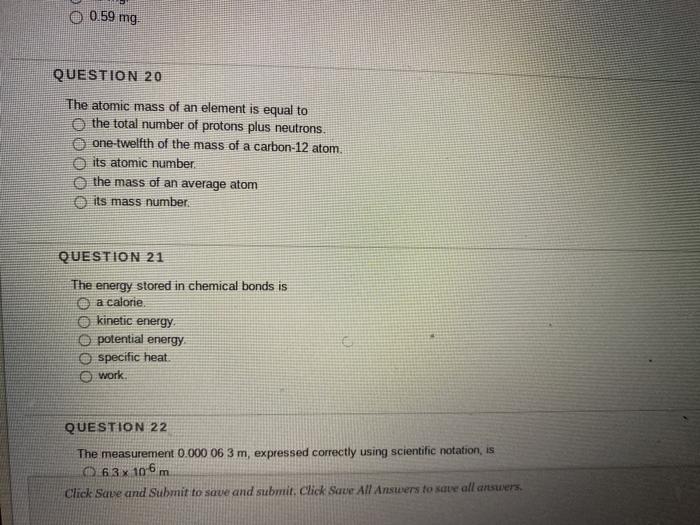
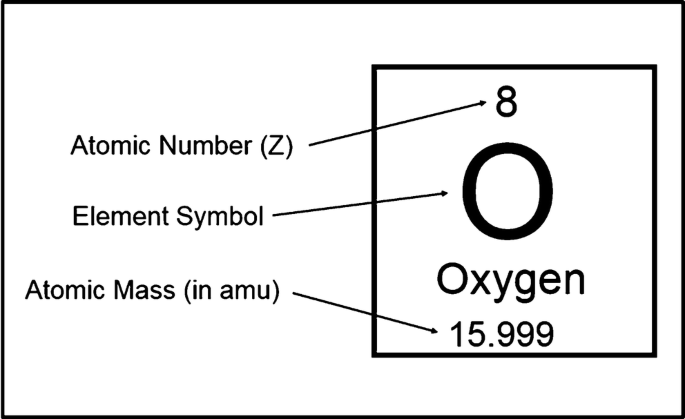
Atomic Number & Mass Number | How to Find the Atomic Mass Number - Video & Lesson Transcript | Study.com

Therefore: There are 3 subatomic particles: protons, neutrons and electrons. These are measured in “ atomic mass units ” ( amu ) as their mass is so small. - ppt download

Chapter 3 Atoms and Elements 3.4 Atomic Number and Mass Number 1 Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition. - ppt download