Which of the following pair of gases will have same rate of diffusion under similar conditions? - YouTube

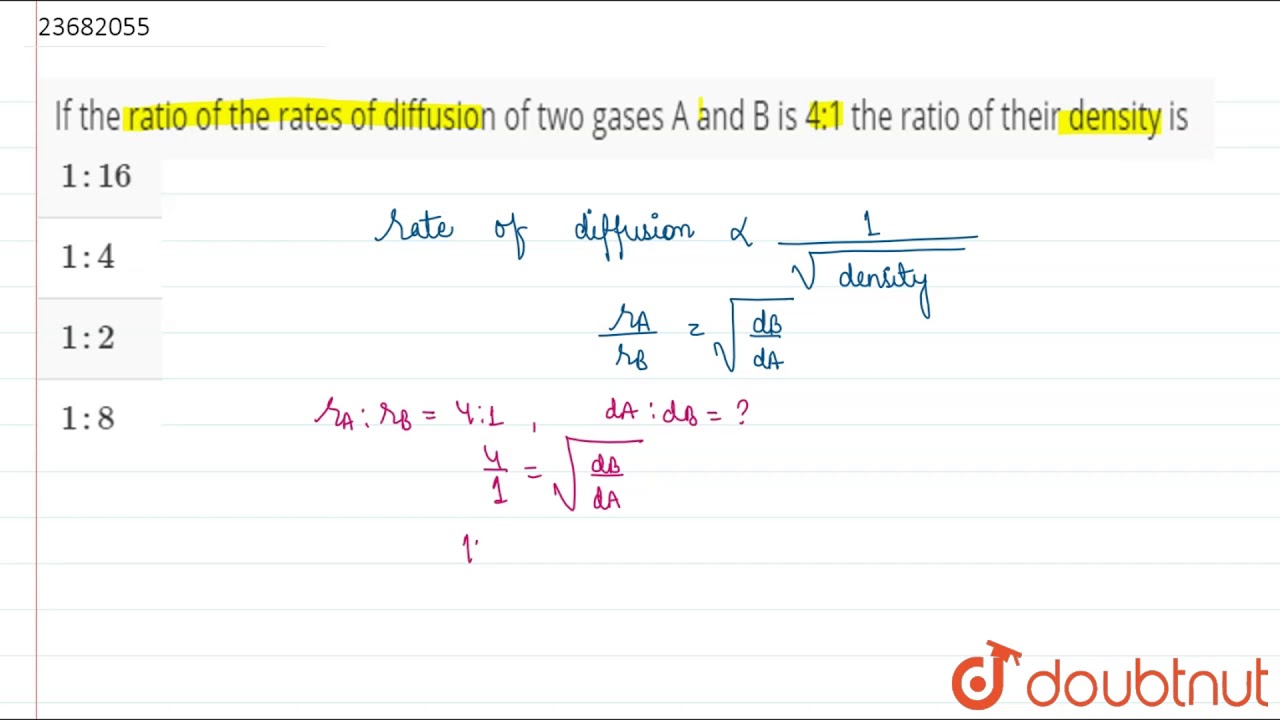
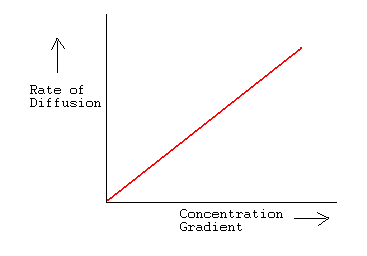
The ratio of rate of diffusion of gases A and B is 1 : 4. If the ratio of their masses present in the mixture is 2 : 3, what is the ratio of their mole fraction ?
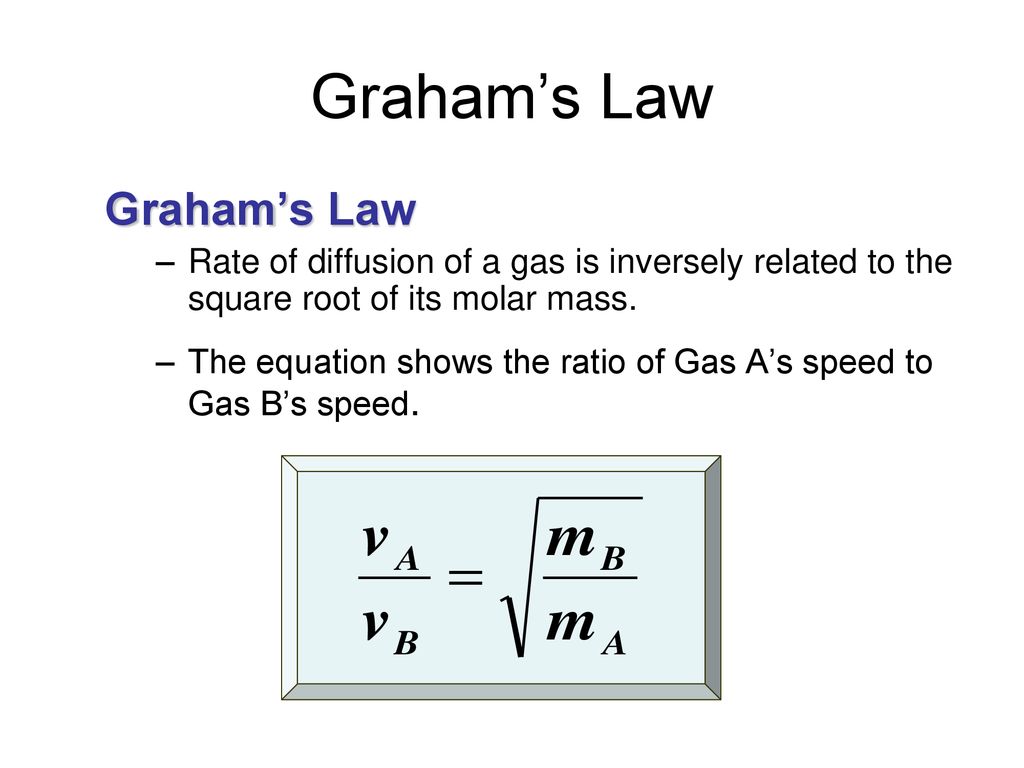
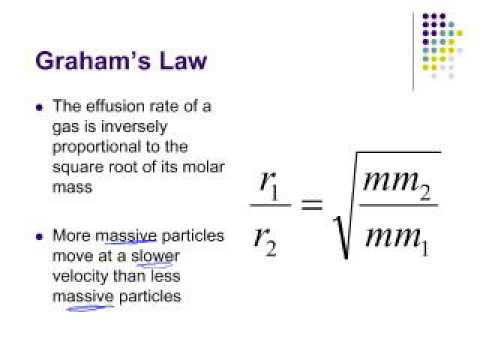
Can the rate of effusion or diffusion be negative, in accordance with Graham's law? If so, how? - Quora
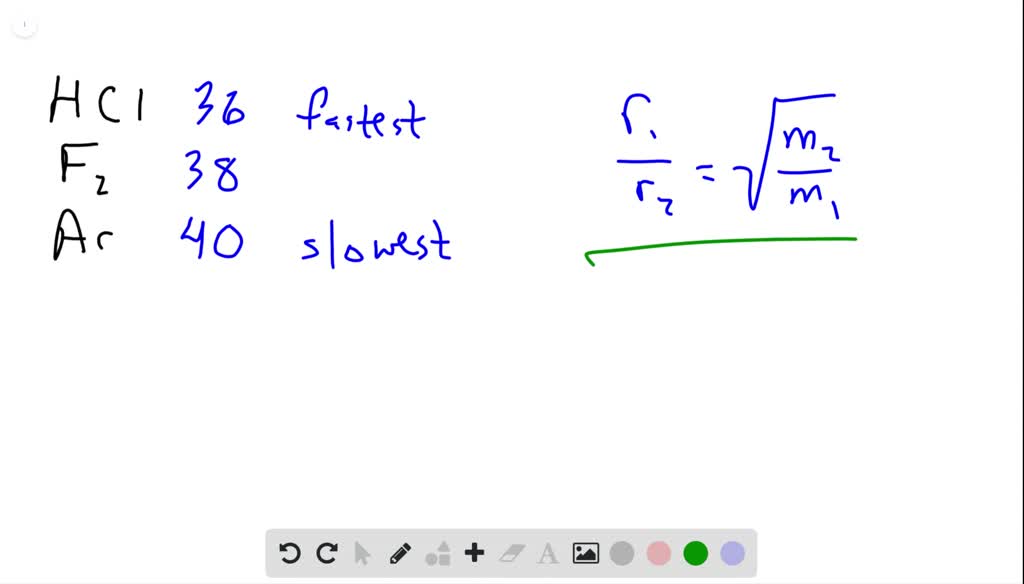
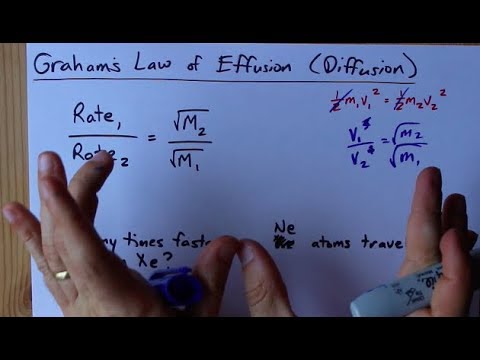
SOLVED:Rank the following gases in order of their speed of diffusion through a membrane, and calculate the ratio of their diffusion rates: HCl, F2, Ar .

Rate of diffusion of gas A is (1)/(2) that of gas 'B'. If molecular mass of gas A is 16 than calculate molecular mass of gas 'B'.

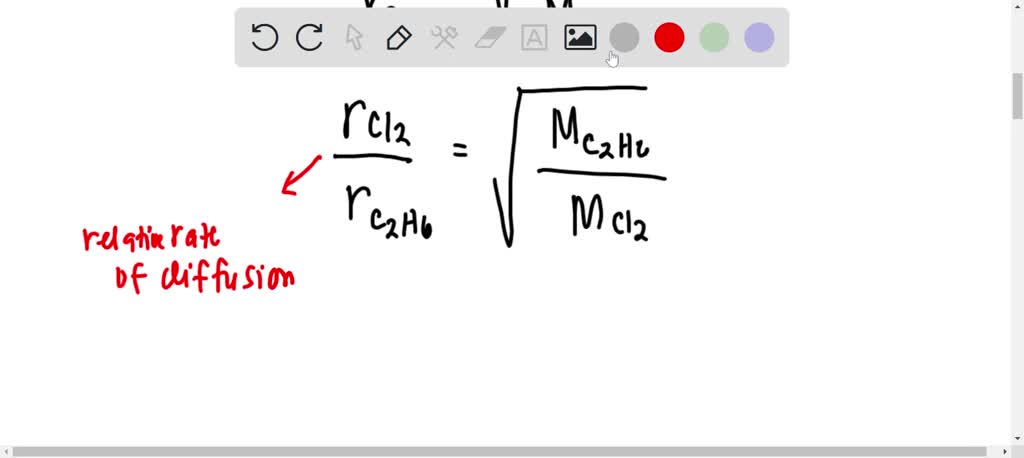
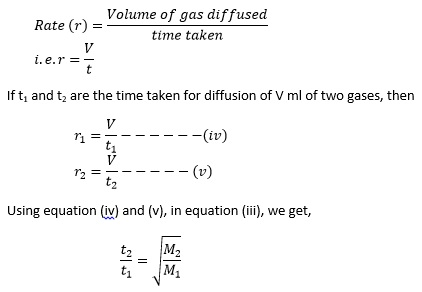
acid base - What is the relative rate of diffusion of ammonia to hydrogen chloride, both in gaseous states? - Chemistry Stack Exchange