
Vague FDA policies on adverse event data are keeping patients from accessing investigational drugs | Fierce Healthcare
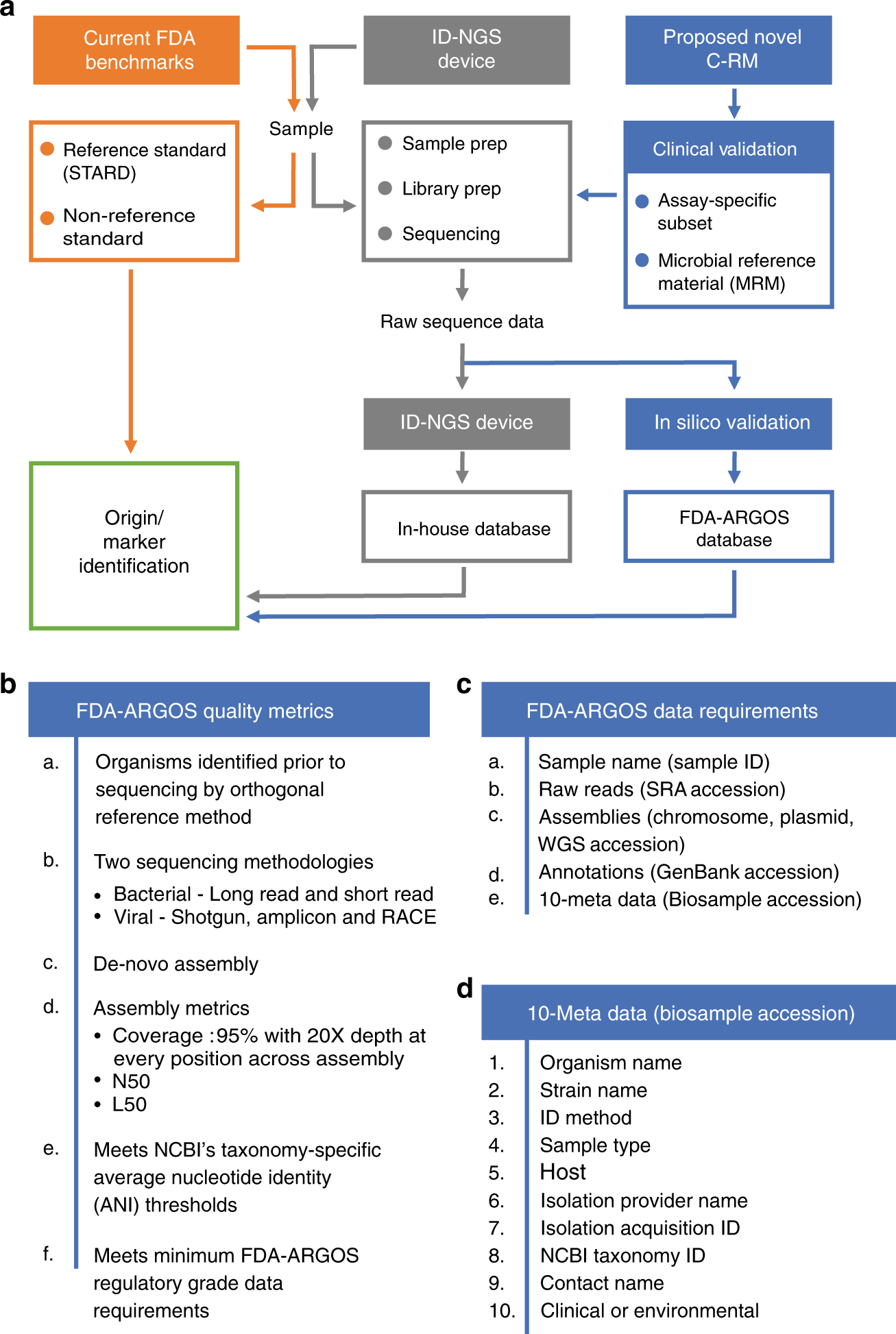
FDA-ARGOS is a database with public quality-controlled reference genomes for diagnostic use and regulatory science | Nature Communications
GAO-17-564, Accessible Version, INVESTIGATIONAL NEW DRUGS: FDA Has Taken Steps to Improve the Expanded Access Program but Should
July 6, 2022 (https://www.accessdata.fda.gov/scripts/foi/FOIRequest/requestinfo.cfm) Food and Drug Administration Division of Fr
![PDF] IDAAPM: Integrated database of ADMET and adverse effects of predictive modeling based on FDA approved drug data PDF] IDAAPM: Integrated database of ADMET and adverse effects of predictive modeling based on FDA approved drug data](https://i1.rgstatic.net/publication/303977127_IDAAPM_Integrated_database_of_ADMET_and_adverse_effects_of_predictive_modeling_based_on_FDA_approved_drug_data/links/5900b6774585156502a00f65/largepreview.png)
PDF] IDAAPM: Integrated database of ADMET and adverse effects of predictive modeling based on FDA approved drug data

19 Printable certificate of analysis fda Forms and Templates - Fillable Samples in PDF, Word to Download | PDFfiller

Transparency advocates win victory for public access to clinical trial data | Center for Science in the Public Interest

Investigational New Drugs: FDA Has Taken Steps to Improve the Expanded Access Program but Should Further Clarify How Adverse Events Data Are Used | U.S. GAO












